Table of Contents
Error

Error is an action which means mistake in analytical chemistry the difference between the true value or a standard value and observed value is called error.
If we talk about error in our language, then we can say that any mistake is called error, in the language of chemistry, error is the difference between a true value and a standard value.
Example:- If a tablet contains a source of paracetamol and after analysis the analyst to observed 490 mg of paracetamol of tablets.
Then, absolute error ✓500-490= 10mg
Sources of error

1. Sample preparations
2. Error by analyst
3. Equipment problem
4. Calibration
5. Reporting error
6. Calculation error
7. Error in method selection
8. Sampling error
9. Laboratory environment
10. Error during transport
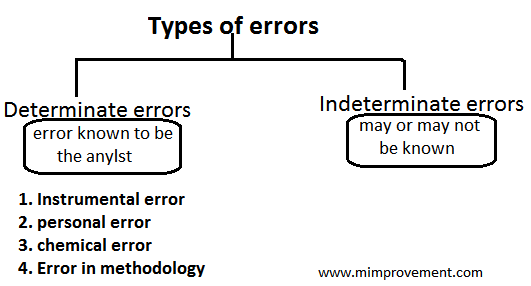
Types of error
There are two types of error:-
1. Determinate error:- The error which is known to be analyst is called as determinate error.
2. Indeterminate error:- Indeterminate errors are often called accidental of random error. They are related by a small difference in series of measurement made by the same analyzed under identical conditions.
Methods of minimizing error
Error can be minimized by understanding the source and type of error. The predictable errors can be minimized by correcting the directly whereas the under predictable error can be minimized by following the standard protocols and good laboratory particles strictly.
Some of the methods to minimized the errors are discussed as follows:-
1. Instrumental error
These errors can be minimized by checking properly the equipment used for the analysis before starting of only analysis.
Proper calibration should be performed to ensure the performance of equipment’s. Faulty equipment should be corrected by the experts and rechecked for accuracy of the results. If the performance is not satisfied then a replacement should be done.
2. Personal error
Skilled returns should be employed or the knowledge of the operators to perform analysis is to be ensured pair to analysis. Regular reporting of analysis can be done.
3. Chemical error
Standards chemical from authentic source with out impurities must be used for analysis.
4. Error in methodology
These errors can be avoided by the following the standard method with proper references.
5. Indeterminate error
Since indeterminate errors are not predictable, the entire procedure of analysis should be carried out in a well planned warmer constituency all factors. which effect the accuracy and precision of results.



Nycc video sirr
You video is really amazing. Thanks for sharing the content.
Dear mimprovement.com admin, Your posts are always well-supported by research and data.
Dear mimprovement.com webmaster, Thanks for the well-organized and comprehensive post!