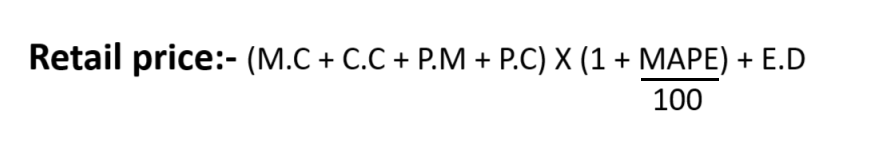Table of Contents
Drug price control order
The drug price control order implements by central government to control and manage the prices of essential medicines. It’s played a major role in ensure that essential medicines are affordable to the general public. The main purpose of DPCO is to prevent unfair pricing practices by pharmaceutical companies.
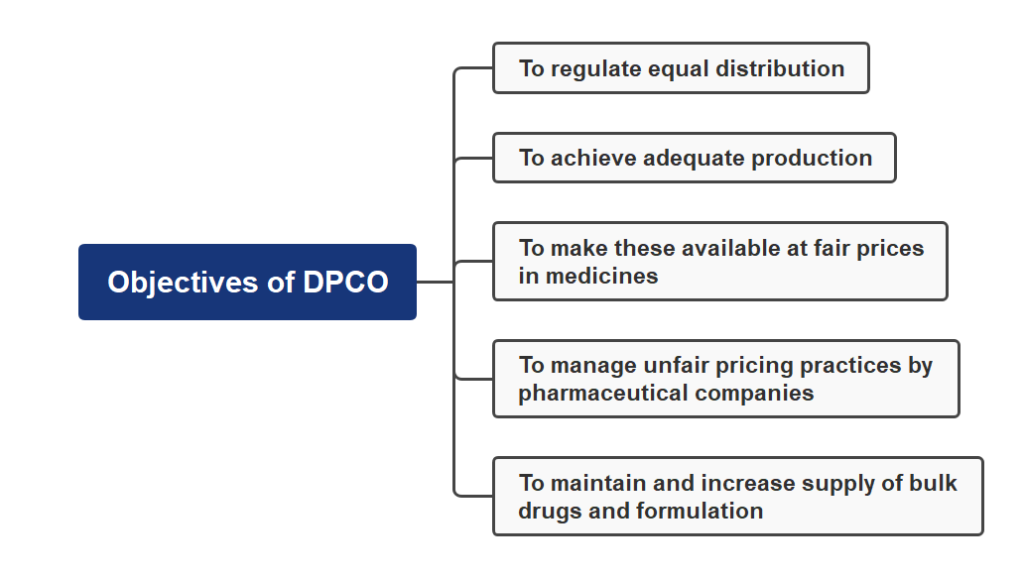
Objectives of DPCO
These are some following objectives of DPCO:
1. To achieve adequate production.
2. To regulate equal distribution.
3. To maintain and increase supply of bulk drugs and formulation.
4. To make these available at fair prices in medicines.
5. To manage unfair pricing practices by pharmaceutical companies.
Schedules for DPCO
The drug price control order has following schedules:
1. Schedule-1: This schedule contains the list of 74 bulk drugs
2. Schedule-2: This schedule contains various forms for approval or revision of prices of scheduled formulations.
3. Schedules-3: It specifies the maximum pre-tax return on sales turnover of manufacturers importers of formulations under A, B & C categories.
History of DPCO
These are line by line history of drug price control order:
1. The drug price control order
2. Replaced by DPCO 2013, confirmed by section 3 essential commodities act 1955.
3. In 1966, parliament members felt that manufactures charging high rate on drugs.
4. To control on high drug rates, DPCO 1966 was passed under section 3 of essential commodities act 1955.
5. DPCO 1966 replaced by DPCO 1970.
6. In 1974, Hathi committee was formed and submitted its report in 1975.
7. DPCO 1970 replaced by DPCO 1979.
8. DPCO 1979 replaced by DPCO 1987.
9. DPCO 1987 replaced by DPCO 1995.
10. Finally DPCO 1955 replaced by DPCO.
Definitions of some essential points in DPCO
1. Bulk drugs
It means any pharmaceutical, chemical, biological or plant product including its salt ester and derivatives, conforming of pharmacopeia or others standard specified in the second schedule to the drug and cosmetics act 1940.
2. Schedule bulk drugs
It means a bulk drug specified in the 1st schedule.
3. Non-schedule bulk drugs
It means a bulk drug not specified in the first schedule.
4. Schedule formation
It means a formulation containing any bulk drug specified in the first schedule either individually or in combination with other drugs, including one or more than one drug or drug not specified in the first schedule except single ingredient formulation based on bulk drug specified in first schedule and sale under the genetic name.
5. Retail price
It means the retail price of a drug arrived at or fixed in accordance with the provision of this order and includes ceiling price.
Retail price formulation
These are following formula shall be used to determine retail price:
R.P: Retail price
M.C: Material cost
C.C: Conversion cost
P.M: Packing material cost
P.C: Packing charge
MAPE: Maximum allowable post manufacturing expenses.
E.D: Excise duty
Sales price of bulk drugs
Government has power to fix the maximum sale price, while fixing the sale price government shall take into following v considerations:
1. post-tax return of 14% on net worth.
2. Return of 22% on capital employed.
3. On the basis of production, post-tax return of 18% on net worth or 26% on capital employed.
Manufacturer fill detail in form-1 for revision of sale price and government will respond within 4 months. Manufacturer will submit information in form-1 & 2 within 30 days. Make necessary inquiry and then government fix maximum sale price of bulk drug notify in official gazette.
Manufacturer to submit information which required
1. Scheduled bulk drugs
A list of all schedule bulk drugs produced by him within 30 days of the commencement of this order and indicate the details of the cost of each of such bulk drug in form-1. The detail of the cost of each schedule bulk drug, which has been produced after the commencement of this order in form-1 by the 30th September every year.
2. Non-schedule bulk drug
A list of such bulk drug produced by him within 30 days of the commencement of this order and indicate the details of the cost of each of such bulk drug in form-1. The details of the cost of each non-schedule bulk drug produced by him including such bulk drugs, which has been produced after the commencement of this order in form 1 by the 30th September every year.
Retail price of schedule formulation
A manufacturer can’t increase such prices fixed by government without the prior approval of the government for revision of the retail price of scheduled formation, a manufacturer can apply to the government.
Prior approval of the price by the government is also essential to market a new pack or a new formulation or new doses form of existing schedule formulation of manufacturer or importer. No person shall sell or dispose of any imported schedule formulation without prior approval of its price from the government.