Table of Contents
Atomic structure and atomic model
Atomic theory
Every substance in the world is made up of some small atom, this information was first given by Kanad in India, whereas John Dalton gave this information widely.
John Dalton gave detailed information about the atomic structure (for the first time); hence he is also called the father of atomic structure. John Dalton named the word atom and said that atom can’t be broken. In India, Kanad had given this information for the first time but Kanad could not explain it to the people. There are many such phenomena in the world, such as Satyendra Nath Bose had given the theory of the five states of matter, but he could not explain it to the people, when later Einstein explained it to the people, then it was published so that its name is become Bose- Einstein condensate.

According to John Dalton, the atom cannot be broken, but in modern times the theory of Dalton was proved wrong and the atom was broken into electrons, protons, neutrons, neutrinos, mesons, pi-mesons, etc. Thus, an atom is mainly made up of electrons, protons and neutrons. Neutrons and protons are found in the nucleus of an atom while electrons revolve outside.
Fundamental particles of an atom
A particle which does not require any other particle to form it is called a fundamental particle. As electron is a fundamental particle whereas nucleus is not a fundamental particle because nucleus is made up of neutrons and protons. There are two types of fundamental particles of an atom: permanent fundamental particles and temporary fundamental particles. The permanent fundamental particles present in an atom are following: electrons, protons and neutrons. Temporary fundamental particles are not necessarily present in the atom such as mesons, pie mesons, neutrinos, etc.
Shape of atom
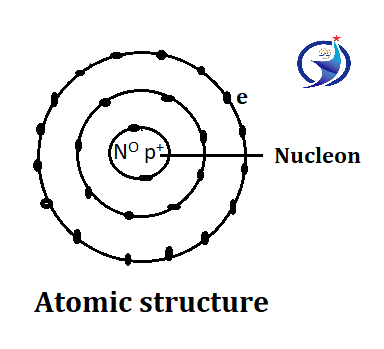
The shape of an atom is spherical with electrons revolving in its outer layer. The center of the atom is called the nucleus, the nucleus was first discovered by Rutherford. The particle found inside the nucleus is called a nucleon which is made up of protons and neutrons, the electron is not a nucleon because it revolves outside the nucleus. The nucleus of an atom is positively charged so that the total mass of the atom is found in the nucleus. Atomic mass can be measured by measuring the mass of the nucleus, it means, the mass of an atom is equal to the mass of neutron and proton.
The radius of an atom is measured by Angstrom, which is 1A = 10-10m and the radius of the nucleus is measured by Fermi, which is 1f = 10-15m, it is very small. The atomic radius is 1 lakh(105) times greater than the radius of the nucleus.
Atomic model

The model in which the positions of nuclei, electrons, protons and neutrons are shown is called atomic model. The first atomic model was given by J.J. Thomson, he considered the atom like a watermelon, hence the theory is also called Watermelon Theory. According to this, the red part of the watermelon is proton while the electrons are scattered like a watermelon seed. The position of the proton he had stated was quite different from the reality.
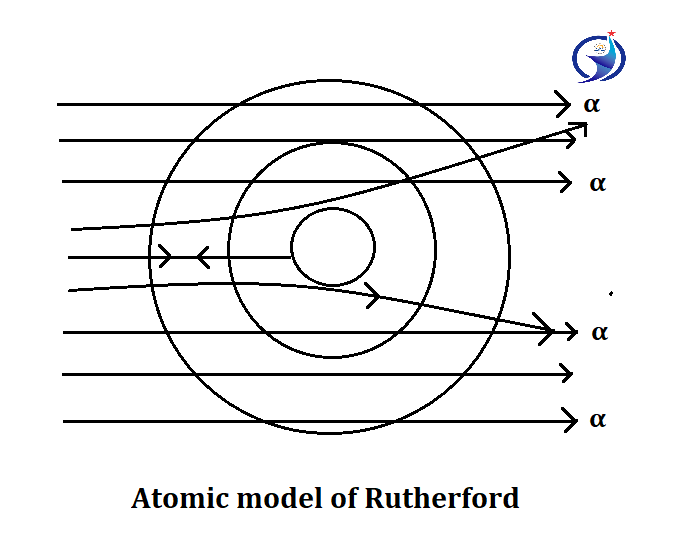
Atomic model of Rutherford
This model is also called α scattering model. In this, Rutherford had extracted α ray from radium and struck a thin layer of gold and gave the following information:
1. Most of the rays have passed through the gold sheet, so most of the atom is hollow.
2. Some α rays passed from the central part of the atom through a slight deviation (oblique), so he said that the central part of the atom is positive.
3. One of the 20,000 α-Ray came back after hitting the middle of the atom, so he said that the central part of the atom is solid, which he named the nucleus.
According to Rutherford, the proton resides in the nucleus of an atom while the electron revolves outside.
Barbary model
This model is recognized, it was given by Nilsborough. According to them, the nucleus is in the center of the atom while the electron revolves outside in a circular orbit. These are collectively called the nucleon. When the electron revolves in its original orbit, it does not emit energy, that is, there is no change in its energy. When an electron moves from the nucleus to a distant orbit, that is, from a lower orbit to a higher orbit, it acquires energy from an external source, that is, its energy increases. When an electron moves from an orbit away from the nucleus to an orbit near the nucleus, it emits energy, that is, its energy decreases.
Electron, Proton and Neutron
Electron:- It was discovered by J.J. Thomson did it in 1897 AD. It is also called cathode ray. There is a debt charge on it. Absolute mass– When the mass of a particle is determined without comparing it to any other, it is called absolute mass. Its absolute mass is 9.1 × 10-31kg. The electron has a 1.6 × 10-19C charge.
Proton:- It is also called anode ray. It was discovered by Goldstein in 1919 AD. While the naming was done by Rutherford. There is money in it. Its absolute mass is 1.6725 × 10-27kg. The charge on the proton is +1.6 × 10-19 C.
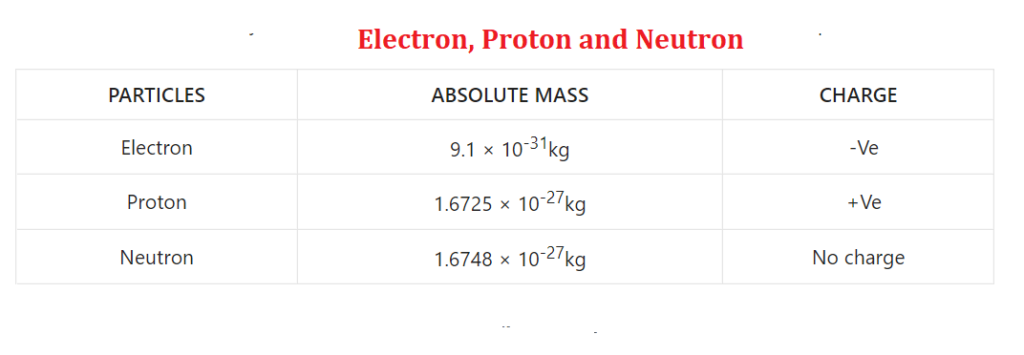
Neutron:- It was discovered by Chadwick in 1932 AD. There is no charge on this. That’s why it took a long time to find it. There is no passion on it. Its absolute mass is 1.6 × 10-27 kg. The charge on a neutron is 0C.