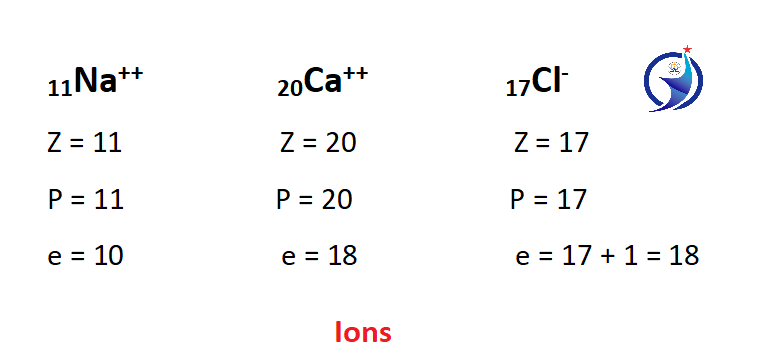Table of Contents
Atomic number and atomic weight
The number of protons present in the nucleus of an atom is called atomic number; it was first discovered by Moseley. The atomic number is denoted by ‘Z’. The atomic number of any element is equal to the number of protons or electrons present in it. (Z = P = e–).
The number of electrons in an atom is equal to the number of protons, but the number of electrons cannot be called atomic number because the number of electrons in an atom keeps on decreasing.
Atomic number
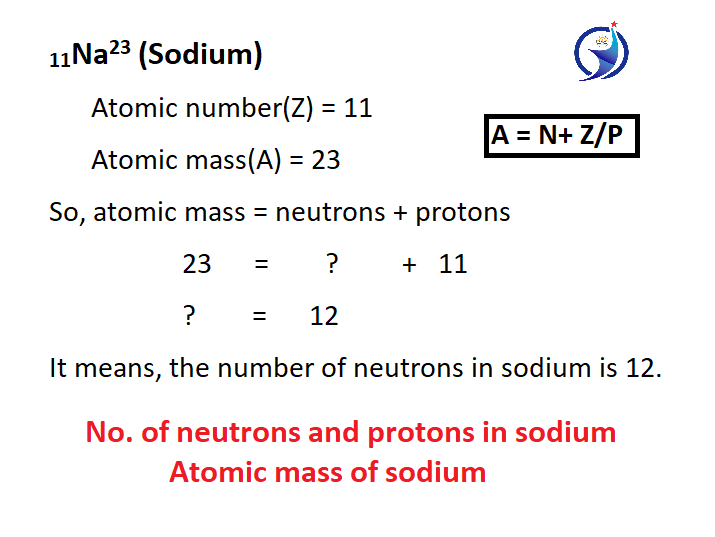
When an atom is written, the number written below it is called atomic number and the number written above the atom is called atomic weight. As you can see in the example. The atomic weight of an atom is equal to the sum of the protons and neutrons present in its nucleus.
As shown in the example, the atomic number of sodium is 11 and its atomic weight is 23. If the atomic weight of sodium is 23, it means that the sum of the number of neutrons and protons present inside sodium is 23 as you know. The number of protons present in sodium is 11 because itself the atomic number is 11, then to find the number of neutrons present in sodium, the number of protons of sodium is subtracted from the atomic weight, so that the number of neutrons present in sodium is known.
Ions
An atom that has either received or donated an electron. An ion has a charge, an atom has a positive charge for donating an electron and a negative charge for receiving an electron. The atomic number of an ion does not change but the number of electrons changes.
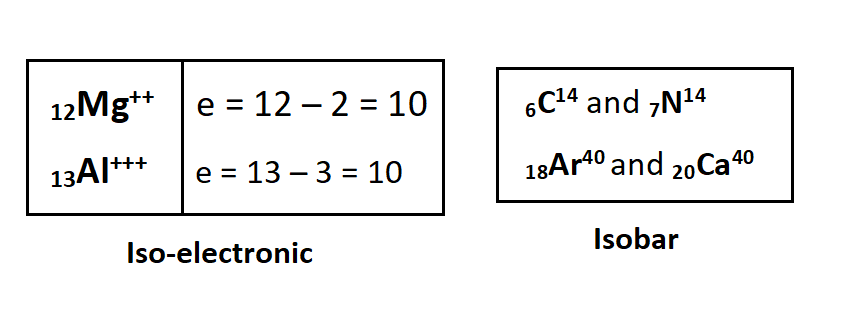
Isoelectronic, Isobar and Iso-tones
Elements that have the same number of electrons are called isoelectronic and those elements which have the same atomic weight are called isobars. Elements that have the same number of neutrons in their nucleus are called iso-tones.
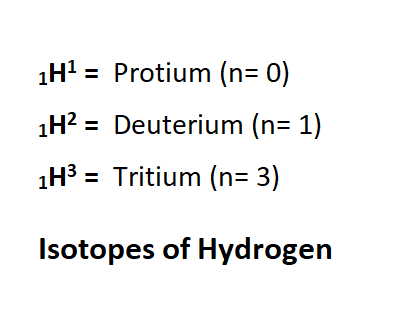
Isotopes
Elements that have the same atomic number are called isotopes that have the same number of protons but different mass numbers. Due to the difference in the number of neutrons in isotopes, the mass number also varies.
Uses of isotopes
1. Carbon-14 (C14) is used to determine the age of fossils.
2. u235 is used to determine the age of rocks.
3. Fe59 (iron) is used in a disease called anemia.
4. AS 74 is used in the treatment of tumors (cancer).
5. Co 60 (cobalt) is used in the treatment of cancer.
Orbit or shell
At the center of the atom is the nucleus, outside which the electrons revolve around the circular path which the electrons revolve around, the same circular path is called the orbit. It is denoted by K, L, M, N….. The K orbit is closest to the nucleus as is the orbital. As the distance from the nucleus increases, the energy level also increases.
When an electron moves away from the nucleus, its energy increases, that is, it acquires energy, but when it comes towards the nucleus, its energy decreases. But when it remains in its original orbit, there is no change in its energy.
According to the Bohr–Bury scheme, the number of electrons in an orbital is based on 2n2, where n is the number of orbitals.
Orbits
A cloud of electrons forms outside the orbit. which is called an orbital. The number of orbitals is half the number of electrons in the sub-orbital. An orbital can have a maximum of two electrons. Electrons fill the orbital according to Hund’s law. According to this law the electrons enter the orbital first one by one. After that a large pair of electrons in the opposite spin starts.