Table of Contents
Anthracene
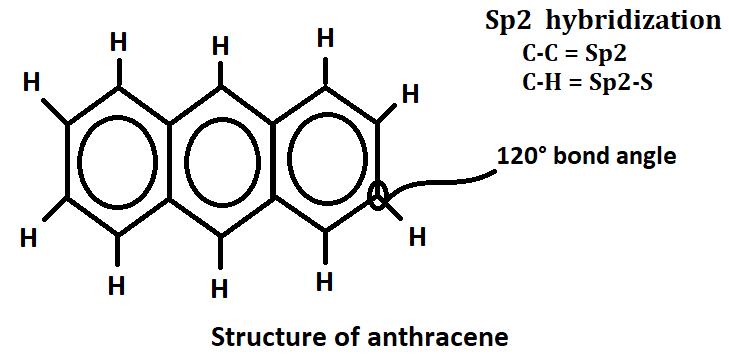
Anthracene composed of three fused benzene ring and it is obtained from coal tar. The molecular formula of anthracene is C14H10 and molecular weight is 178. It is a colorless solid polycyclic aromatic hydrocarbon and shows blue fluorescence in UV light.
Structure of anthracene
It has Sp2 hybridization and the Sp2 hybrid orbitals overlap with each other and with S-orbital of the ten-hydrogen atom faming C-C and C-H bonds.
Resonance of anthracene
These are different bond length between carbon-carbon bond. Resonance energy of anthracene is 84 Kcal/mole (average 28Kcal/mole per ring), which is lower than benzene so anthracene is much less aromatic than benzene.
Synthesis of anthracene
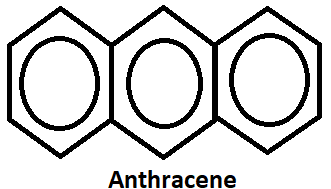
1. By Friedel craft reaction:- Anthracene is prepared by condensation of two molecules of benzyl chloride in the presence of aluminum trichloride(AlCl3).
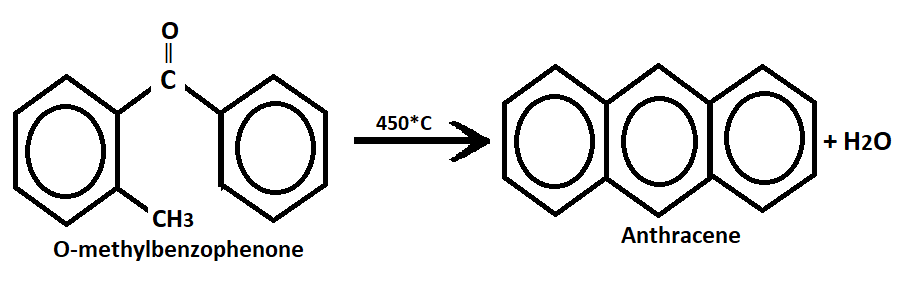
2. By Haworth synthesis:- This involves the treatment of benzene with phthalic anhydride in the presence of aluminum chloride to form O-benzoyl benzoic acid. Then O-benzoyl benzoic acid heated with concentrated sulphuric acid(H2SO4) to give 9,10-anthraquinone. Note distillation of the anthraquinone with zinc dust yield anthracene.
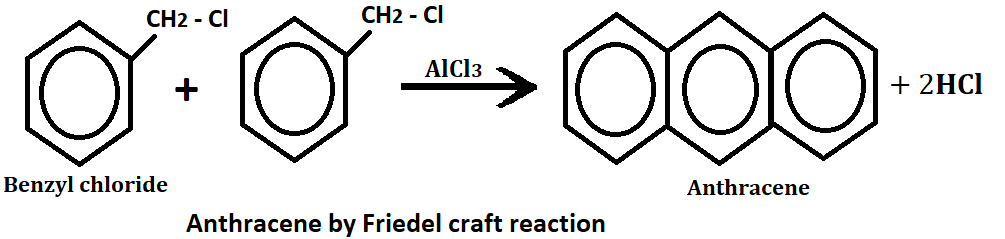
3. By Elb’s reaction:- The conversion of a di-aryl ketones containing a methyl or methylene group to the carbonyl function is known as the elbs reaction.
Chemical reaction of anthracene
There are some important reactions of anthracene-
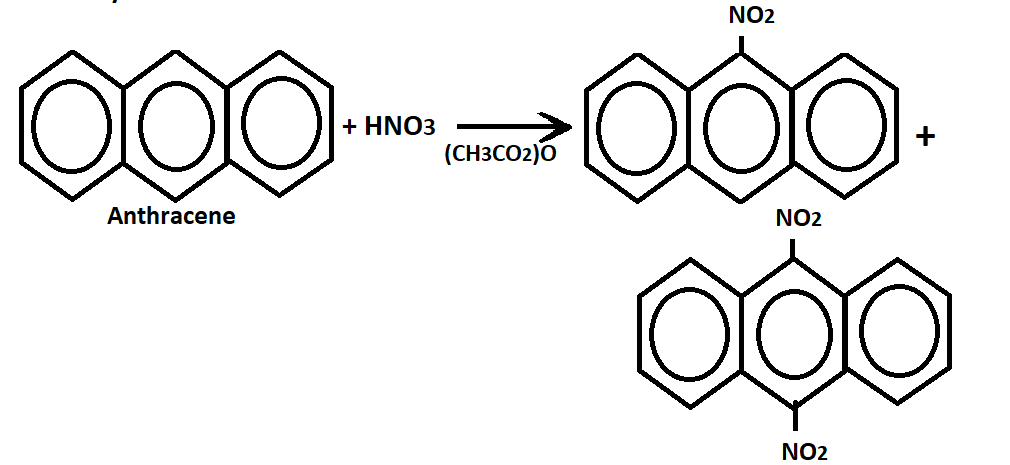
1. Nitration:- Anthracene react with concentrated nitric acid in acetic anhydride(H2SO4 not used here) at room temperature to yield a mixture of 9-nitroanthracene and 9,10-dinitroanthracene.
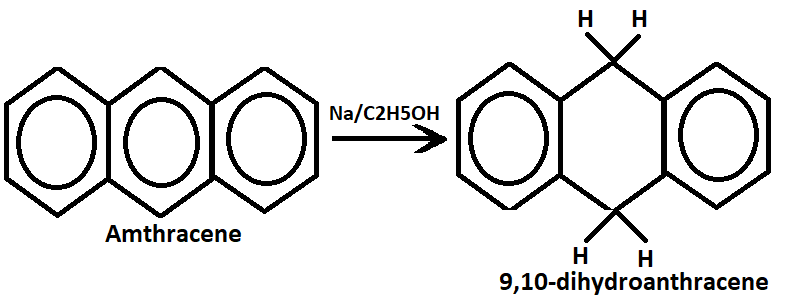
2. Reduction:- Anthracene undergoes reduction with sodium and ethyl alcohol to form 9,10-dinitroanthracene.
3. Oxidation:- Anthracene undergoes oxidation with sodium dichromate and sulfuric acid to form 9,10-dinitroanthracene.
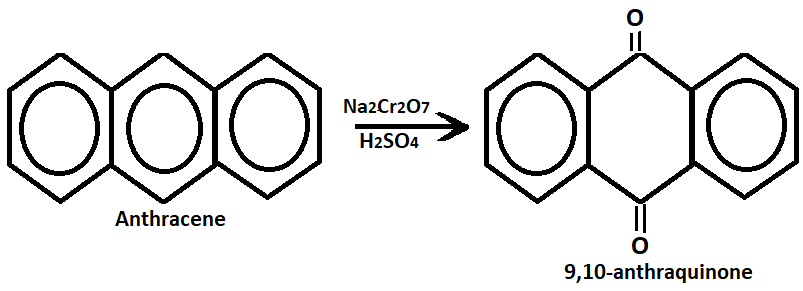
Physical properties of anthracene

1. It is colorless solid.
2. It has melting point of 218°C.
3. It has a boiling point of 340°C.
4. Insoluble in water and soluble in benzene.
5. Chemical formula is C14H10.
Uses of anthracene
1. Used in the manufacture of alizarin and several other dyes.
2. Used as a preservative in wood
3. Used as an insecticide for crops.
4. Also used in anti-cancer agent.
5. It is used as scintillator.

Hi mimprovement.com owner, Your posts are always well-formatted and easy to read.