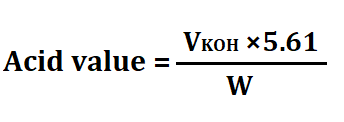Table of Contents
Analytical Constants of Fats and Oils
In this article, we are going to explain analytical constants of fats and oils.
Introduction
In the realm of food science and nutrition, fats and oils play a vital role. They are not only a source of energy but also contribute to the texture and flavor of our favorite dishes. Analytical constants are crucial parameters used to characterize fats and oils, providing essential information for various applications, from food processing to quality control. In this article, we will delve into the world of analytical constants of fats and oils, exploring their significance and applications.
Understanding Analytical Constants
Before we dive into the specifics, let’s first grasp the concept of analytical constants. Analytical constants are measurable values that define the chemical and physical properties of fats and oils. They serve as benchmarks for quality assessment, aiding in the identification and differentiation of various types of fats and oils.
The Main Analytical Constants of fats and oils
1. Iodine Value (IV)
The iodine value measures the degree of unsaturation in fats and oils. It quantifies the number of grams of iodine that can be absorbed by 100 grams of the fat or oil. Oils with higher IVs contain more unsaturated fatty acids.
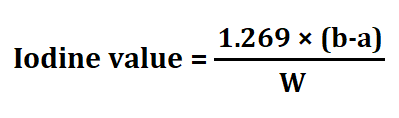
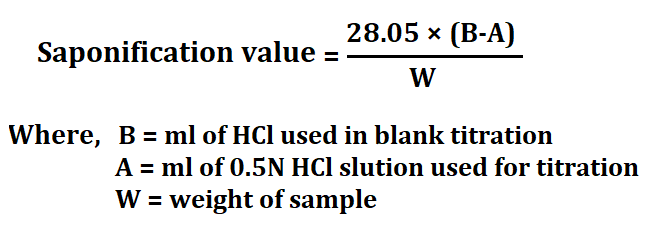
2. Saponification Value (SV)
The saponification value indicates the average molecular weight of the fatty acids present in fats and oils. It is defined as the number of milligrams of potassium hydroxide (KOH) required to saponify one gram of fat or oil. SV is crucial for assessing the purity of fats and oils.
3. Acid Value (AV)
The acid value measures the amount of free fatty acids present in fats and oils. It is determined by titrating the sample with a base and is expressed as milligrams of potassium hydroxide (KOH) required to neutralize the acids in one gram of the sample.
4. Peroxide Value (PV)
Peroxide value reflects the extent of oxidation in fats and oils. It measures the amount of peroxides present, which are formed during the initial stages of oxidation. A high PV indicates the potential for rancidity.
5. Refractive Index (RI)
The refractive index is a measure of how much light is bent, or refracted, when it passes through a substance. In the context of fats and oils, it can help determine the purity and composition. Different types of fats and oils have distinct refractive indices, making it a valuable tool for identification.
6. Specific Gravity (SG)
Specific gravity measures the density of a substance compared to the density of water. It provides insights into the overall composition of fats and oils. For example, fats with a high specific gravity may contain a higher percentage of saturated fats.
7. Melting Point
The melting point of fats and oils is an essential parameter in various applications. It defines the temperature at which a solid fat transitions into a liquid state. Melting points are crucial in food processing, as they determine the texture and mouthfeel of products like chocolate and margarine.
8. Solid Fat Content (SFC)
Solid fat content is vital for the food industry, especially in the production of baked goods and confections. It quantifies the percentage of fat that remains solid at a specific temperature. Understanding SFC helps manufacturers achieve the desired texture in their products
Applications of Analytical Constants of fats and oils
Analytical constants find applications in various fields, including:
1. Food Industry
In the food industry, these constants are used to determine the quality and stability of fats and oils used in cooking, baking, and frying. It ensures that the products meet safety and taste standards.
2. Pharmaceutical Industry
Pharmaceutical companies rely on analytical constants to assess the suitability of fats and oils for drug formulation. Properly chosen fats and oils can enhance the absorption of certain medications.
3. Cosmetics
In the cosmetics industry, these constants help formulate skincare products. They ensure the desired texture, appearance, and stability of creams, lotions, and makeup.
4. Biodiesel Production
Analytical constants are crucial in the production of biodiesel, as they determine the quality of the feedstock oils used.
Factors Affecting Analytical Constants of fats and oils
Several factors can influence the analytical constants of fats and oils:
1. Source of Fats and Oils
The type of plant or animal source greatly impacts the composition of fats and oils. For instance, olive oil differs significantly from palm oil in terms of IV and SV.
2. Processing Methods
The methods used to extract and refine fats and oils can alter their analytical constants. Cold-pressed oils, for example, retain more natural compounds and have different constants than heavily processed oils.
Quality Control and Certification of fats and oils
Analytical constants are also instrumental in quality control and certification processes. Regulatory bodies and industry standards often specify acceptable ranges for these constants. This ensures that consumers receive safe and consistent products.
Environmental Impact
The analytical constants of fats and oils are not only relevant to human consumption but also have environmental implications. For instance, the saponification value is essential in assessing the environmental impact of fats and oils in wastewater, as it determines their potential to cause pollution. (Analytical constants of fats and oils)
Emerging Trends
As technology advances, new methods and instruments for analyzing fats and oils continue to emerge. Spectroscopy, chromatography, and mass spectrometry are some of the cutting-edge techniques used to obtain precise analytical data. These innovations contribute to a deeper understanding of the composition and properties of fats and oils.