Table of Contents
Phenol
Phenol is an aromatic organic compound in which one hydroxyl group(OH) replace one hydrogen(H) atom in benzene. Phenol is an organic compound with the molecular formula C6H5OH.
Phenol is a colorless, oily liquid that smells like ether and has a slightly sweet taste. It is miscible in water and most organic solvents. Phenol is not soluble in gasoline, kerosene, or diesel fuel.
Phenols are used as disinfectants and antiseptics because of their low cost, efficacy against both gram-positive and gram-negative bacteria, lack of significant toxicity to humans, and ease of use. They are also used as preservatives for both food and wood products such as plywood used in construction.
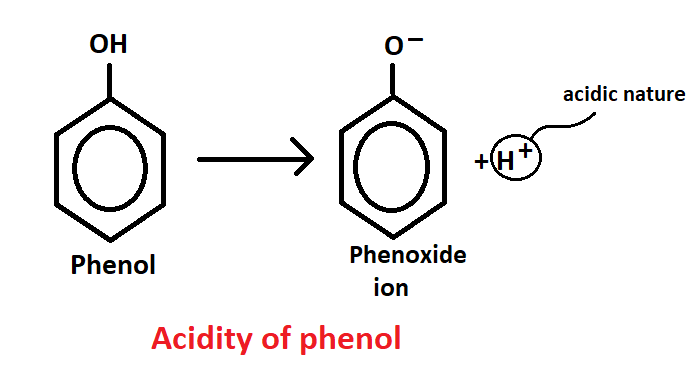
Acidity of phenol
When any substance dissociated into H+ ion then it shows acidic nature and if any substance dissociated OH- ion then it shows basic nature.
So, when phenol is dissociated it break down in two parts in which first is H+, due to this phenol is acidic is nature and second part is phenoxide. Phenol is a very acidic compound, with a pH of 2.
The substance or compound which replace H+ ion or quick have more acidic. The substance which is stable after H+ ion release, that substance is equally acidic.
Why phenol is acidic in nature
The substituent also affects how acidic phenol is because it can change where the hydrogen atom attaches to the molecule. For example, if there is an H-C-OH group and an H-C-OCH3 group, then the H-C-OH will be more acidic than if it were replaced by an H-C-OCH3.
Phenol is acidic in nature because of the presence of a hydroxyl group (-OH) and a benzene ring. This makes it different from alcohols which are neutral or basic in nature.
We also know the which substance is more stable after releasing of H+ ion has more acidic. We can easily check the acidity of stability of any compound by these two methods-
1. Resonance
2. %S character
1. Resonance of phenol:- The resonance of phenol is the frequency at which it will absorb energy from an electromagnetic wave. So, phenol has resonance, it is stable due to resonance then it is acidic in nature.
2. %S character of phenol:- Phenol is also known as carbolic acid, hydroxybenzene, or phenic acid. It is a colorless oily liquid with an unpleasant smell. Phenol has many industrial and commercial uses. For example, it can be used in the production of plastics and herbicides.
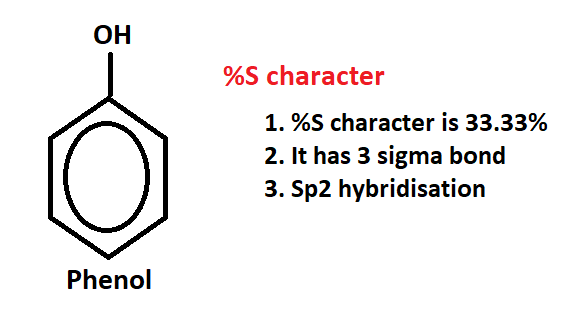
So, phenol has 3 sigma bonds then it has sp2 hybridization in which %S character is 33.33%, which is more acidic than alcohol due to this reason too phenol is more acidic than alcohol.
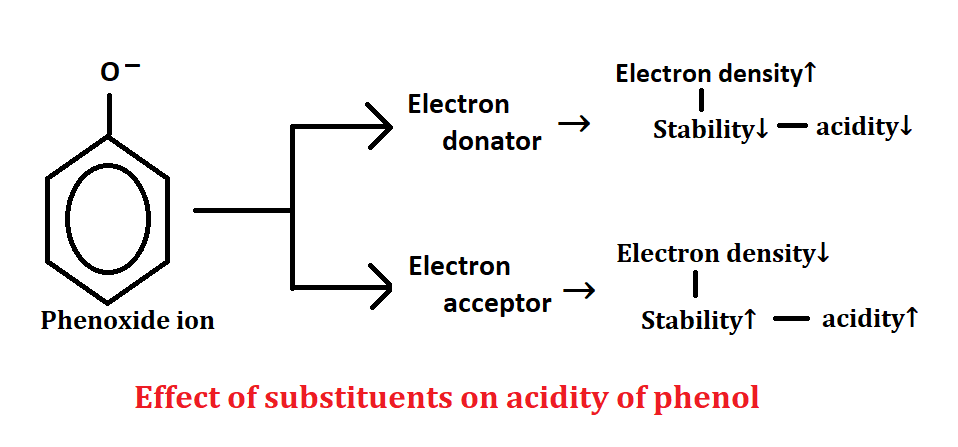
Effect of substituents on acidity of phenol
There are two types of substituents group which will add or attached on phenol: electron donator group and electron acceptor group.
1. Electron donator group:- which has the ability to donate electrons. like CH3, Cl, OH, etc. If the phenol ion donates electrons, then the electron density increases. When the electron density of a compound increases, it becomes unstable, and when a compound becomes unstable, its acidity decreases.
2. Electron acceptor group:- which has the ability to accept electrons. like CN, NO2, NH2, etc. If the phenol ion accepts an electron, then the electron density decreases. When the electron density of a compound decreases, it becomes stable, and when a compound is stable, its acidity increases.
Qualitative test for phenols
These are some methods with the help of which qualitative test of phenol can be done easily:-
1. Litmus test
2. Liebermann’s test
3. Ferric chloride test
4. Bromine water test
5. Phthalein dye test
1. Litmus test of phenol
The litmus test is a chemical indicator. It has the properly of turning red in the presence of an acid and blue in the presence of an alkali.
2. Liebermann’s test of phenol
Liebermann’s test is a sample chemical test that identifies the presence of phenol in a sample. Phenol is acidic and will react to the metal ions in the solutions.
When phenol is reacted with NaNO2 and concentrated H2SO4, a deep green colour is obtained which change into red when dilute with water. When a little amount of NaOH added to it, the solution becomes deep blue colour.
3. Ferric chloride test of phenol of phenol
Ferric chloride test is a chemical test for phenol. Phenol is an organic compound that has a strong odor and is used in the production of many products, such as plastic, solvents and pharmaceuticals. The ferric chloride to a solution of phenol.

Aqueous solution of phenols reacts with freshly prepared ferric chloride solution give colored complex. Mostly phenols given dark color.
4. Bromine water test of phenol
The bromine water test is a qualitative test that is used to identify phenols in water. Phenol is organic compound that are found in many neutral and man-made substance. They can be found in the environment, food and pharmaceuticals.

Take aqueous solution of phenol and add excess of bromine water. A yellowish white precipitate is obtained.
5. Phthalein dye test(fluorescein test) of phenol
Phenol on heating with phthalic anhydride in the presence of concentrated sulphuric acid(H2SO4) it forms colorless condensation compound called phenolphthalein.
On further reaction with dilution sodium hydroxide(NaOH) it gives pink color fluorescent compound called fluorescein. (Which indicate that substance is phenol.)