Table of Contents
Geometrical isomerism
The isomerism which arises due to restricted (frozen) rotation about double bond in molecules. It refers to the phenomenon of molecules having the same atoms, but different spatial structures due to their double bonds. This type of molecule can be either cis or trans, and can cause a variety of physical and chemical properties in them. The position of the double bonds even affects how light interacts with molecules.
The configuration of the isomeric 2-butene are structure 1 & 2 and these configuration are differentiated in their names by the prefix Cis (Latin – in this side) & trans(Latin – across), which indicate that the methyl are on the same side or on the on the opposite side of the molecules. In cis-isomer the methyl group are present close enough together & causes crowding. It is expected that cis-isomer will be less stable than the trans-isomer. In this type of isomerism, the compounds have same molecular formula but differ in their geometry. This is generally due to restricted rotation around the double bond atom. When all four groups are different, cis isomer is the one which the groups containing the longest carbon chain are on the same side of the double bond.
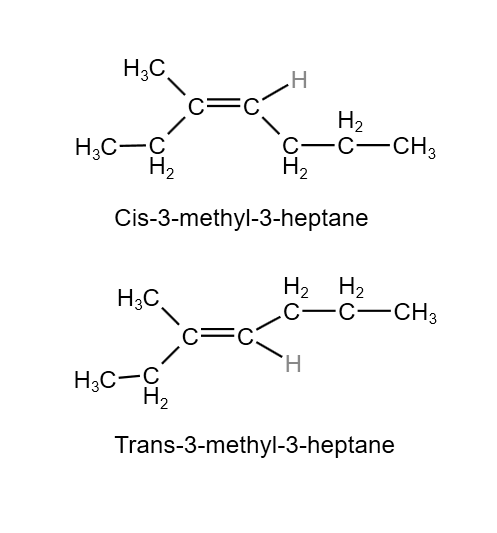
Geometrical isomerism will not be possible if one of the 2 carbons atoms forming the double bond carries the two identical groups. The only requirement for geometrical isomerism is that substituent are each carbon are different.
Condition for geometrical isomerism
1. There should be restricted rotation about a bond in the molecules.
2. Both substituents on each carbon about which rotation is restricted should be different.
Nomenclature of geometrical isomerism
Geometrical isomer can be nomenclature by three methods:
1. Cis-trans nomenclature
Compound show this nomenclature due to restriction rotation about carbon-carbon double bond. Compounds should have at least one double bond between carbon bond. At least one similar atom between both double bonded carbons.
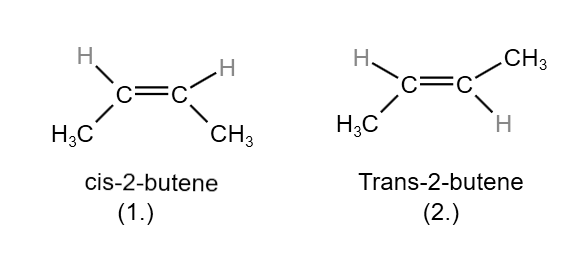
Cis:- the isomer in which the identical groups are on the same side of the double bond.
Trans:- the isomer in which the identical group are on the opposite sides of the double bond.
2. E-Z nomenclature
This number of geometrical isomers is more general and can be applied to all compound.
Z (Zusammen – together)
E (Entegegen – opposite)
It is used on Cohn-Ingold system (sequence rule). The group of highest priority on each carbon atom is identified by using the sequence rules.
For this nomenclature, the two groups are attached to each carbon of the double bond are assigned priority. The priority is given according to RS system of configuration or sequence rule. If two higher priority groups are on opposite sides of the double bond the prefix ‘E’ is used and if the two higher priority group are same side of the double bond the prefix ‘Z’ is used. Thus, the compound (A) will be (Z)-1-bromo-1-chloro-2-iodoethylene & compound (B) will be (E)-1-bromo-1-chloro-2-iodoethylene.
Rules for priority
1. Higher the atomic number, higher will be priority. Among isotopes, the one with higher mass number get the higher priority.
2. If two same identical atoms are attached to the stereocenter then the relative priority of the two group is decided by the comparing next atom in the groups.
3. Syn-Anti nomenclature
This type of nomenclature (isomerism) shows those compound which have at least one C=N or N=N. The compound showing this type of isomerism are aldoximes, ketoximes and phenylhydrazone. In compound, where nitrogen form a double bond with carbon atom, the 3 valences of nitrogen atom are not coplanar. Aldoximes & ketoximes exist in 2 geometrical isomeric form, there is no free rotation about the =C=N-, the origin contains these groups (=C=N-OH) in their structure.

The term syn and anti are used instead of cis & trans in geometrical isomerism of compound having carbon and nitrogen bond. In aldoximes which have H & OH groups. The H & OH atom on the same side is called syn form while when these groups are an opposite sites to the configuration is Anti form.
Method of determination of configuration of geometrical isomerism
There is no general method for determining the configuration of geometrical isomerism. The choice of method depends upon the nature of the compound. At the same time, the use of several methods will give more reliable results. The following methods are used for compound that as geometrical isomerism due to the presence of double bond and also due to cyclic structure.
1. Physical methods
The melting point and intensity of absorption of the cis isomer are lower than those of the trans. The boiling point, solubility, heat of combustion, heat of hydrogenation, density, refract index, dipole moment and dissociation constant of the Cis-isomer are greater than those of Trans. So, by comparing these properties compound identified as cis or trans.
2. Cyclisation methods
A cis-isomer undergoes cyclisation much more readily than the trans isomers. Wislicenus suggested firstly that intramolecular reaction is more likely to occur the closer together the reacting group are in the molecule. The principal is always true for reaction in which having but don’t hold for elimination reaction in which double out triple bond is produced.
Example: – Malic acid form maleic anhydride but fumaric acid doesn’t form fumaric anhydride, it is due to the fact that cis isomer is expected to undergo cyclisation much more readily than trans.
3. Conversion methods
In this case, the configuration of pairs of geometrical isomers by determine by converting them into compound whose configuration already known.
Example:- there are two trichloracrotinic acid and of which can be hydrolyzed to fumaric & therefore, this must be trans isomer & other must be cis-isomer.


Dear mimprovement.com owner, Your posts are always well-timed and relevant.
To the mimprovement.com admin, Keep sharing your knowledge!