Table of Contents
Gaseous and liquid states
Gaseous state
The gaseous state is characterized by the mobility of the molecules. The kinetic energy of the molecules is large and they move about freely in all directions.
Liquid state
The liquid state is characterized by a relatively small amount of kinetic energy for each molecule. The molecules are not free to move about but can only vibrate about fixed positions in the liquid.
The liquid state is characterized by a relatively small amount of kinetic energy for each molecule. The molecules are not free to move about but can only vibrate about fixed positions in the liquid.
The solid state is characterized by a large amount of kinetic energy for each molecule. The molecules are free to move about in the solid. This article is Gaseous and liquid states.
Arrangement of molecules in a solid state
The arrangement of molecules in a solid is even closer than in a liquid, and they are not able to move around at all. This is why solids have a definite shape, whereas liquids and gases do not. Gases are easily compressible because the molecules are not strongly attracted to each other and can move around freely.
Arrangement of molecules in a liquid state
Liquids are less compressible because the molecules are attracted to each other and tend to stay in place.
Solids are the least compressible because the molecules are tightly packed together and cannot move at all. This is why solids have a definite shape and volume. The state of matter is determined by the temperature and pressure. At high temperatures, the molecules have more energy and move around more. This is why gases are more common at high temperatures. At low temperatures, the molecules have less energy and move around less. This is why solids are more common at low temperatures. This article is Gaseous and liquid states.
Arrangement of molecules in a gaseous state
The pressure is determined by the number of molecules in a given area. The more molecules there are, the higher the pressure. This is why gases are more common at high pressures. The key difference between gaseous and liquid states is in the way the molecules are arranged. Gases have molecules that are spread out evenly and are constantly moving around, while liquids have molecules that are closer together and are moving less. This article is Gaseous and liquid states.
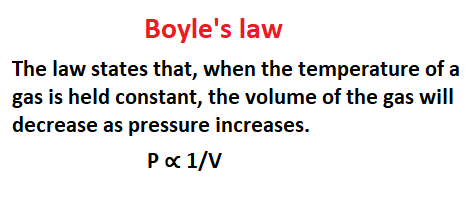
Boyle’s law
The law states that, when the temperature of a gas is held constant, the volume of the gas will decrease as pressure increases.
This law is named after Robert Boyle, an Irish physicist and chemist who lived in the 17th century. He discovered this law by studying how gases behave at different temperatures and pressures. This article is Gaseous and liquid states.
Charles’s law
Charles’s law is a gas law that relates the volume of a fixed mass of gas to its temperature. The law states that the volume of a fixed mass of gas is directly proportional to the absolute temperature. The equation for Charles’s Law is V ∝ T.

A gaseous state is when a substance is in the form of a gas. There are two types of gaseous states: atmospheric and solution. The atmospheric gaseous state is when the substance exists in the air as a gas because it has not been compressed. The solution gaseous state is when the substance has been dissolved in a liquid and then evaporates to make it into a gas.
Charles’ Law states that if you increase the temperature of an object, its volume will also increase, but if you decrease the temperature, its volume will decrease. This article is Gaseous and liquid states.
Avogadro law
At the molecular level, there are two states of matter: solid and liquid. These are the only two states in which molecules tend to stay close to each other and maintain their shape. Gaseous state is a third state in which molecules have enough kinetic energy to escape from each other’s attraction.
The Avogadro law is a formula that describes the relationship between the number of atoms or molecules in a sample of gas at a given temperature and pressure, and the volume of gas needed to contain them. The law was named after Italian physicist Amedeo Avogadro, who published it in 1811. This article is Gaseous and liquid states.
Combined gas law
The combined gas law is a gas law that combines the ideal gas law and the perfect gas law.
The combined gas law is a combination of the ideal gas law and the perfect gas law. The combined gas law can be used to find pressure, volume, temperature, and amount of substance in any situation involving gases. This article is Gaseous and liquid states.
Ideal gas law
Gaseous and liquid states of matter are states that are diametrically opposed to each other, yet they share the same properties.
The ideal gas law is a simple equation that defines the relationship between the pressure, volume, and temperature of a gas. The equation is PV=nRT where P is pressure in atmospheres, V is volume in liters, n is number of moles of gas R is the universal gas constant and T is temperature in Kelvin. This article is Gaseous and liquid states.
Uses of ideal gas law
This law can be used to calculate various properties of gases. For example, if you know the pressure and temperature for a given mass of gas you can use this equation to calculate its density or if you know its density and volume you can use it to calculate its specific heat capacity or even its speed at which it will diffuse through a membrane. Gaseous and liquid states
Kinetic theory of gases
The kinetic theory of gases is a theory that explains the behavior of gases in terms of the motion of the particles that make up the gas. The theory was first proposed by Daniel Bernoulli in 1738, and it has been refined over the years to become the modern theory that we know today. The kinetic theory of gases is based on the assumption that the molecules that make up a gas are in constant motion. The molecules move in a random way, and they collide with each other and with the walls of the container. The collisions between the molecules and the walls of the container cause the pressure that we observe in a gas.
The kinetic theory of gases can be used to explain many properties of gases, such as the pressure-volume relationship, the behavior of gases in mixing, and the diffusion of gases. The theory can also be used to calculate the properties of gases, such as the speed of sound in a gas, the viscosity of a gas, and the heat capacity of a gas. This article is Gaseous and liquid states.
Molecular collisions
Molecular collisions are ubiquitous in the universe. They occur when atoms or molecules collide with each other. The vast majority of collisions are elastic, meaning that the molecules bounce off of each other without undergoing a chemical reaction. However, some collisions are inelastic, meaning that the molecules stick together after the collision. Inelastic collisions are important because they can lead to chemical reactions. For example, when two atoms of hydrogen collide, they can form a molecule of H2. This reaction is the basis of the hydrogen bomb.

Molecular collisions also play an important role in the formation of stars and planets. When a cloud of gas and dust collides, the resulting heat and pressure can cause the molecules to fuse together, forming a new star. This article is Gaseous and liquid states.
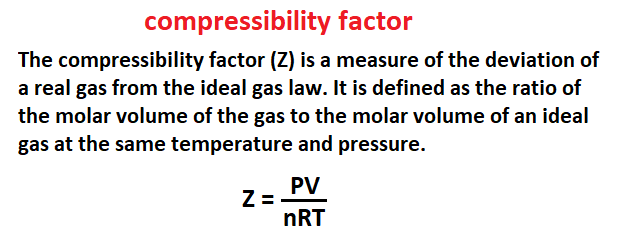
Compressibility factor
The compressibility factor (Z) is a measure of the deviation of a real gas from the ideal gas law. It is defined as the ratio of the molar volume of the gas to the molar volume of an ideal gas at the same temperature and pressure.
The compressibility factor is important in the study of real gases because it allows the properties of real gases to be predicted from the properties of ideal gases. The compressibility factor is also important in the design of compressors and other equipment that is used to compress gases.
The compressibility factor is usually determined experimentally by measuring the molar volume of the gas at different pressures and temperatures. The compressibility factor can also be calculated from the equation of state of the gas. Gaseous and liquid states