Table of Contents
Introduction of alkaloids
Alkaloids are basic nitrogenous compound of plants or animals’ origin and possesses a pharmacological action. They contain nitrogen and mainly in heterocyclic ring. It is mainly derived from amino acids and founds in animals, fungi & bacteria.
These are some drugs
1. Atropine
It is a tropane alkaloid derived from the Atropa belladonna plant, also known as deadly nightshade. Family of atropine is Solanaceae. Atropine blocks nerve impulses and leading to dilation of the pupils and an increase in heart rate (tachycardia). It’s used to treat conditions like bradycardia (slow heart rate) and as an antidote for certain types of poisoning.
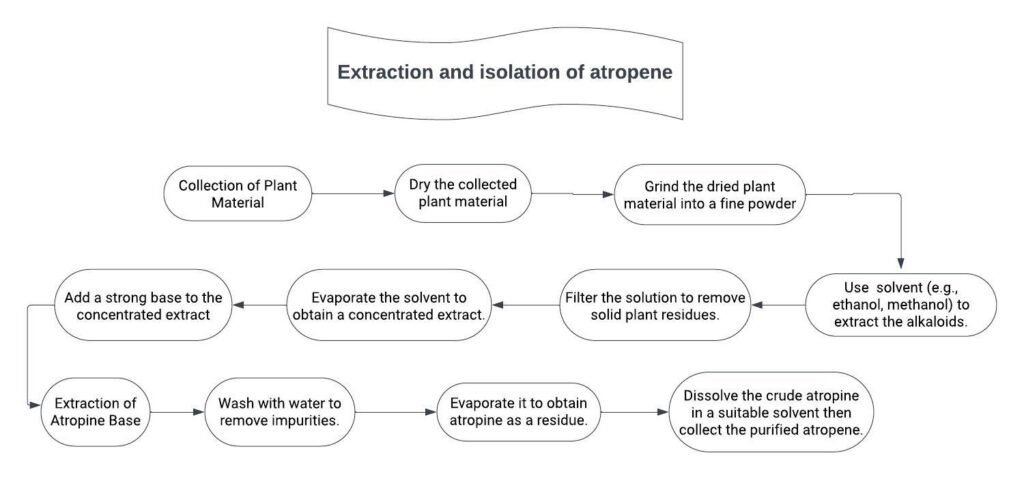
Extraction and isolation
1. Take weight quantity of coarse powder and moisten with sodium carbonate solution.
2. Extract the blended mixture in petroleum ether then filter it.
3. In filtrate, add aqueous acetic acid then extract it with ether.
4. Both are separated through separating funnel and separate aqueous fraction.
5. In aqueous fraction, add sodium carbonate to make solution alkaline.
6. These forms, precipitate of tropane alkaloids.
7. Filter the precipitate and dry to obtain residue.
8. Dissolve the residue in diethyl ether.
9. Filter and concentrate the filtrate.
10. Atropine crystal will be separated out.
11. Filter the crystal and dissolve in alcohol containing sodium hydroxide solution, recrystallize the atropine sulphate from acetone.
12. Separate the crystal of atropine.
Identification test for atropine
Vitali-morin test, Take small quantity of solid atropine s and add 2 drops of concentrated nitric acid in an evaporating dish and evaporated to dryness on water bath.
Then dissolve the residue in 1ml of acetone. Add several drops of a freshly made potassium hydroxide solution in alcohol. Violet colour obtain for to tropane nucleus.
2. Reserpine
It is an indole alkaloid obtained from the root of Rauwolfia serpentina plant. It is soluble in alcohol, acetone and chloroform. Family of reserpine is Apocysnaceae. Reserpine was historically used to treat high blood pressure (hypertension) by depleting certain neurotransmitters. However, due to its side effects and the availability of more effective medications, its use has declined.
Extraction and isolation
1. Rauwolfia root powder is exhaustively extracted with 90% alcohol by percolation.
2. The alcoholic extract is concentrated and dried under reduced pressure below 60°C to yield to Rauwolfia dry extract.
3. Rauwolfia dry extract is extracted with ether-chloroform 90% alcohol (20:8:2.5).
4. Collect the extract and add little dilute ammonia with intermittent shaking. Add water and allow the drug to settle after vigorous shaking.
5. Filter off the solution and extract, the residue with 4 volumes of 0.5N ammonia sulphate is separating funnel.
6. Combine all the extract.
7. The extract is made alkaline with dilute ammonia to liberate alkaloid. Finally extracted with chloroform.
8. Collect the chloroform extract, concentrated and evaporator water bath to yield total Rauwolfia alkaloids.
9. Residue is subjected to column chromatography fraction for the separation of reserpine.
Identification test
Reserpine, when treated with perchloric acid and then heated with sulfuric acid, produces a blue-green coloration. With ferric chloride, reserpine gives a green coloration.
3. Caffeine
Caffeine is found in varying quantities in the seeds, leaves, and fruits of some plants, most commonly in coffee beans, tea leaves, cocoa beans, and cola nuts. Caffeine serves as a stimulant for the central nervous system. It can briefly counteract fatigue and enhance alertness. Recognized as the world’s most frequently ingested psychoactive compound, it’s a fundamental ingredient in coffee, tea, and numerous sodas. The family to which caffeine belongs is Theaceae.
Extraction and isolation
1. The powder tea leaves is extracted with boiling water and the aqueous extract is filtered while hot.
2. The warm extract is treated with bad acetate to precipitate tennis and filtered.
3. The filtrate is treated with excess of dilute sulphuric acid to precipitate lead in the form of lead sulphate.
4. Filter and collect the filtrate.
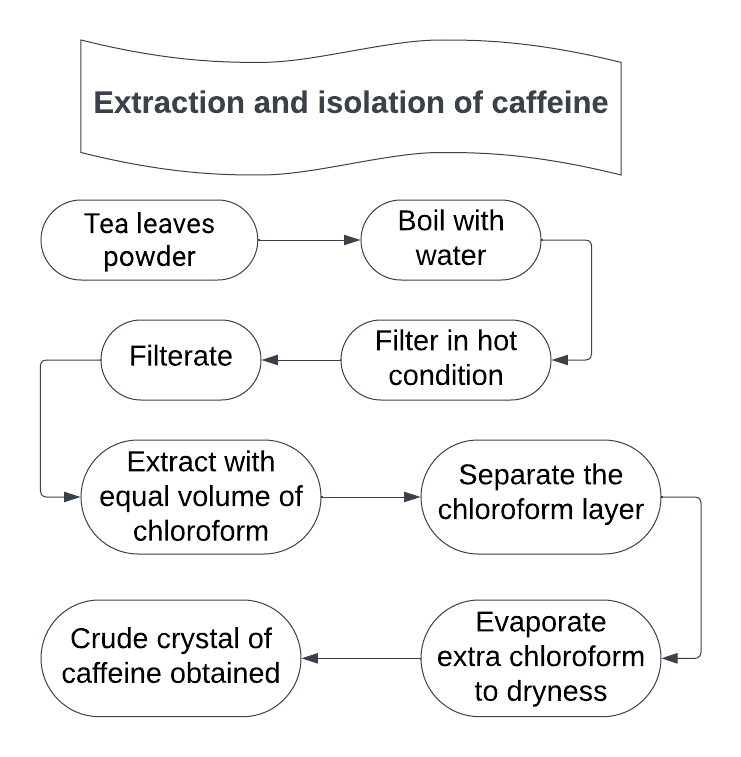
5. The filtrate is boiled with activated charcoal to remove colouring matter, if any filtered to remove charcoal.
6. The filtered decolourised solution is extracted with chloroform successively.
7. Combined the chloroform extract evaporate on water bath to yield caffeine.
8. It is crystallized with alcohol.
Identification test
When heated with chloroform and sulfuric acid, caffeine gives a violet coloration.
Murexide Test: Caffeine, when treated with nitric acid followed by ammonia, produces a purple colour.
4. Quinine
It is a quinoline alkaloids of cinchona bark. The other important alkaloids of this drug are quinidine, cinchonine cinchonidine and cinchonamine. It consists of dried inner bark of cinchona tree. Family of this drug is Rubiaceae. Quinine is known for its antipyretic, analgesic (pain- relieving), and anti-malarial properties. Quinine was historically used to treat malaria, although newer medications have largely replaced it for this purpose.
Isolation and extraction of quinine
1. The dry powder bark material is first well mixed with about 30% of its weight of calcium hydroxide or calcium oxide and sufficient quantity of sodium hydroxide solution to make a paste. It’s allowed to stand for few hours.
2. The mass is then transferred to a Soxhlet apparatus and extraction of carried out with benzene.
3. Subsequently the benzene extract is shaken with successive portions of 5% sulphuric acid.
4. The aqueous acid extract is adjusted the pH 6.5 with dilute sodium hydroxide, cool crystal of natural quinine sulphate is formed.
5. These crystals are freed from cinchonine and cinchonidine by repeated recrystallization from hot water.
6. Colouring matter is removed by activated charcoal.
7. Quinine sulphate crystals are dissolved in dilute sulphuric acid and made alkaline with ammonia.
8. Finally washed to remove sodium and ammonium salts and dried to 45-55°C.
Identification test of quinine
Thalleioquin Test: Quinine, in the presence of diluted sulfuric acid and bromine water, produces a green fluorescent solution called thalleioquin.
Herapathite Test: Quinine, when treated with iodine and potassium iodide, forms an iodo-derivative (herapathite) that displays dichroism. It appears green by reflected light and red by transmitted light.